

 |
||||||||||||
 |
||||||||||||
| Ammonia 1 | ||||||
| Ruth Francis-Floyd and Craig Watson | ||||||
Ammonia is a major metabolic waste product from fish. It is excreted across the gill membranes and in the urine. The primary source of ammonia in aquaculture systems is fish feed. When feed is eaten by fish it is metabolized into the energy, nutrients, and proteins used for survival and growth. As with all animals, there is waste produced by these normal metabolic processes. Ammonia is the principal waste product excreted by fish. In trace amounts, ammonia is odorless and colorless, so the only way for an aquaculturist to know if it is there is to test the water. Of all the water quality parameters which affect fish, ammonia is the most important after oxygen, especially in intensive systems. In small amounts, ammonia causes stress and gill damage. Fish exposed to low levels of ammonia over time are more susceptible to bacterial infections, have poor growth and will not tolerate routine handling as well as they should. Ammonia is a killer when present in higher concentrations, and many unexplained production losses have been caused by ammonia. In water, ammonia occurs in two forms, which together are called the Total Ammonia Nitrogen , or TAN. Chemically, these two forms are represented as NH 4 + and NH 3 . NH 4 + is called Ionized Ammonia because it has a positive electrical charge, and NH 3 is called Unionized Ammonia since it has no charge. This is important to know, since NH 3, unionized ammonia (abbreviated as UIA), is the form which is toxic to fish. Water temperature and pH will effect which form of ammonia is predominant at any given time in an aquatic system. The Nitrogen Cycle In aquaculture, a mechanism called The Nitrogen Cycle eliminates ammonia from the water. As ammonia is excreted, it is converted to another compound called Nitrite (NO 2 ). Nitrite is also toxic to fish and is discussed in a separate publication. This change from ammonia to nitrite is accomplished by bacteria called Nitrosomonas . Another group of bacteria, called Nitrobactcr, convert nitrite to Nitrate (NO 3 ). Nitrate is not toxic to fish except at extremely high levels, and can be considered harmless. Nitrate is used by plants, including algae, for food. This constant change from ammonia to nitrite to nitrate is called the nitrogen cycle ( Figure 1 ). 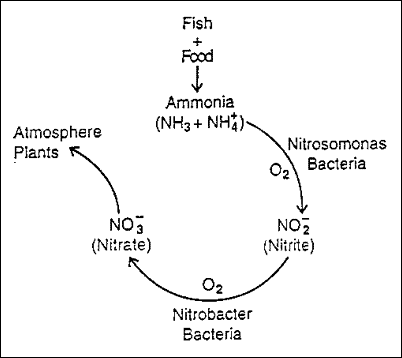
Figure 1: In ponds this process takes place in the surface layers of the mud, but in tanks or aquaria, a special place has to be provided for the bacteria to live and flourish. This is called a biological filter, or biofilter. For further information on biofilters, see Florida Cooperative Service Extension publication FA-12, Principles of Water Recirculation and Filtration in Aquaculture. One important point to mention about the nitrogen cycle, is that both groups of nitrifying bacteria need oxygen to function. If oxygen levels are not sufficient, the process can break down, and you will start seeing levels of ammonia and nitrite rise in the system. more ... |
 |
|||||
| About Us :: Message Board :: Chat | |||||
| Library :: Photo Gallery :: Links & Resources :: Breeders & Sponsors :: Merchandise | |||||
| Website designed by: EthanCote.com | © 2001-2004, SimplyDiscus.com. All Rights Reserved. | ||||